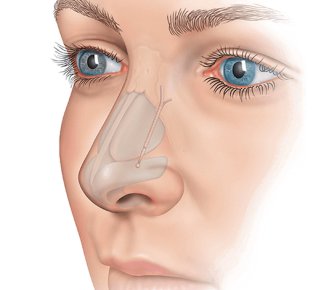
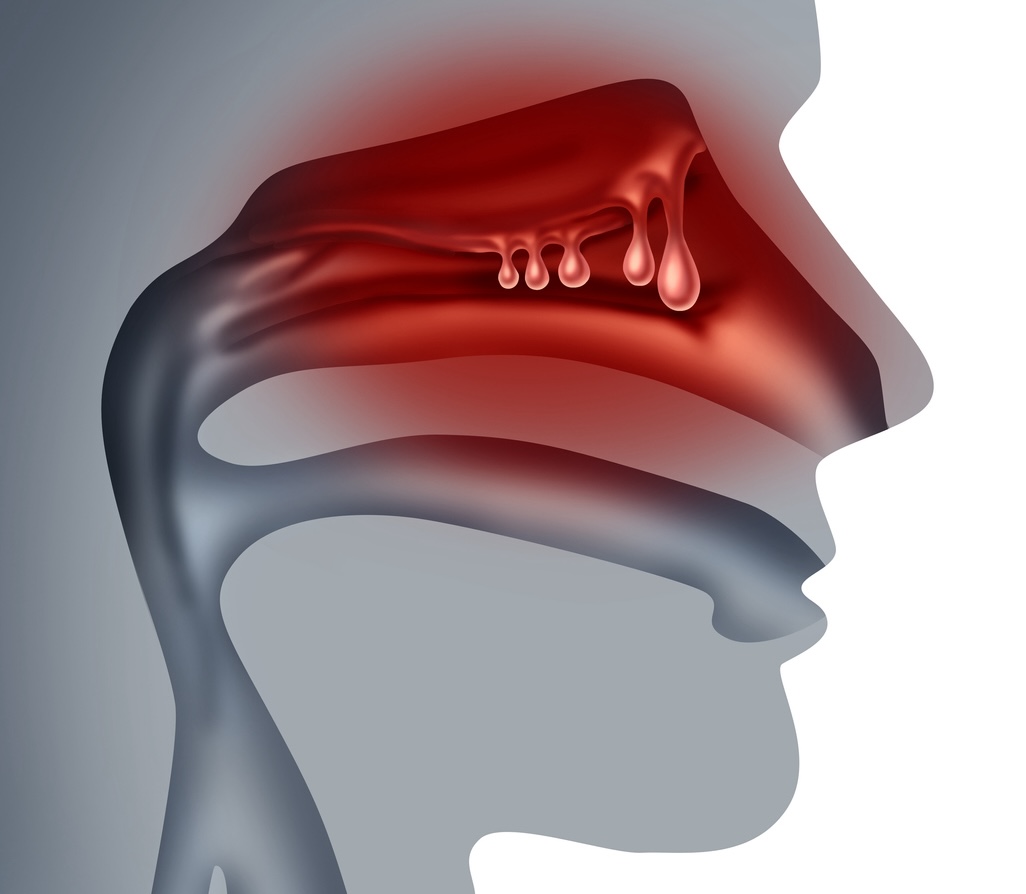
Battling with a Drippy Nose or Constant Congestion?
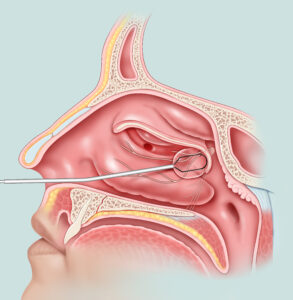
Clarifix
Do you suffer from frequent runny nose, congestion and post-nasal drip even when you aren’t sick? You may have a condition called chronic rhinitis.
Learn more about how Clarifix can help.
Dealing with Obstructive Sleep Apnea?
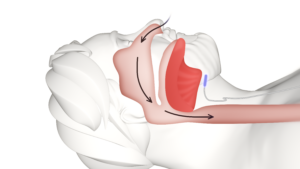
Inspire Procedure
Are you failing to seek relief from using a CPAP machine? Discover the benefits of Inspire Therapy and experience the difference it can make to your sleep.
Learn more today!
Suffering from Pressure & Clogged Ears?
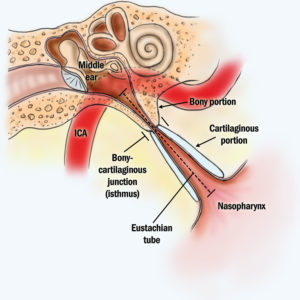
Eustachian Tube Balloon
Do you suffer from fullness and pressure in the ears with inability to clear them?
Learn more about how Eustachian tube balloon dilation can help.
North Atlanta Ear Nose and Throat is excited to announce that the Clarifix, Inspire Procedure and Eustachian Tube Balloon Dilation are simple office procedures that are now covered by Medicare and many commercial insurance providers.


Welcome Aboard: Introducing Our New Physicians Starting June 1st!
Dynamic Duo: Drs. Joseph and Natalie Sciarrino Join NAENTA
Dr. Joseph and Natalie Sciarrino, a husband and wife team of otolaryngologists, are joining NAENTA. Dr. Joseph Sciarrino specializes in otology, sinus and allergy issues, and is a Fellow of the American Academy of Otolaryngic Allergy. Dr. Natalie Sciarrino is a board-certified otolaryngologist with a particular interest in pediatric care and allergy management. Together, they are dedicated to providing comprehensive ENT services to patients of all ages.

We are pleased to have welcomed Dr. Eltahir to North Atlanta ENT.
She is now seeing new patients at our Cumming and Dawsonville locations.
Dr. Hafiah Eltahir is a highly accomplished professional with a diverse background. She has received extensive surgical training in all areas of Otolaryngology, and as a result, she enjoys practicing every facet that the field has to offer. She especially finds joy in treating patients with sinonasal and salivary gland diseases, as well as obstructive sleep apnea (OSA). She is certified in Inspire implantation surgery for OSA and looks forward to growing an Inspire sleep program at North Atlanta ENT and Allergy.
SCHEDULE AN APPOINTMENT


Dear Valued Patient:
The physicians and staff of North Atlanta ENT and Allergy feel privileged that you entrust your care to our practice. The practice will remain open to provide the highest level of care to its patients. We are screening patients depending on the age and medical status of each patient. We are reducing our clinic schedule in an effort to reduce wait times, therefore minimizing the number of patients in our reception area. We ask that capable adults arrive alone and children be accompanied by only one guardian.
North Atlanta Ear, Nose and Throat is committed to your health and safety. Given the current concerns regarding COVID-19 (Coronavirus) and the nature of its transmission; we are asking anyone with the following symptoms to reschedule your appointment. We are not equipped to evaluate, test or treat patients in our clinic for COVID-19 (Coronavirus).
Symptoms include:
- New onset of cough, accompanied by fever
- Fever of 100 or higher
- Shortness of breath
We are also offering telemedicine visits for patients that would like to be evaluated and treated without coming into the office.
For everyone’s safety, all employees will be wearing a mask during your visit.
If you have questions but are unable to come into the office please call us at 770-292-3045 and we will help in any way we can. Our goal is to continue to provide the best care in the safest way possible.
Sincerely,
North Atlanta ENT and Allergy
Top ENT Doctors Serving
Alpharetta, Cumming & Dawsonville

North Atlanta Ear, Nose, Throat, and Allergy has been providing exceptional ENT care to Alpharetta, Cumming, Dawsonville, and surrounding areas for 30 years. Join us on our mission to provide comprehensive, quality, and compassionate ear, nose, and throat care.
Our experienced providers and support staff enables our practice to treat even the most complex medical conditions. We take pride in being a full-service ear, nose, throat, allergy, and sinus specialty office with the capability of handling the most complex cases.
Three Convenient North Atlanta Offices
Meet Our Providers
Meet Our Midlevel Providers
Meet Our Audiology Providers
Practice Highlights
Balloon Sinuplasty (BSP) uses a small, flexible, balloon catheter to open up blocked sinus passageways and facilitate drainage of the mucus that builds up in patients suffering from chronic sinusitis symptoms. Unlike traditional sinus surgery, Balloon Sinuplasty requires no cutting and no removalof bone and tissue.
Testimonials
Larry L. describes his sinus and ear treatment experience with North Atlanta Ear, Nose, Throat & Allergy.
North Atlanta ENT & Allergy Patient Hugh R. Discusses His Experience with Inspire Sleep Therapy.
North Atlanta Ear, Nose, Throat & Allergy Patient Matthew J. Discusses His Sinus Treatment Experience.
Blog and News
Nasal Polyps: Revealing the Basics of This Common Nasal Condition
Nasal polyps are a prevalent nasal condition that affects many individuals worldwide. These non-cancerous growths develop within the lining of the nasal passages and sinuses, causing a range of symptoms that can significantly impact a person's quality of life. Continue reading as we explore the fundamentals of nasal polyps, from…
Understanding the Different Types of Hearing Tests for Young Children
Hearing tests and evaluations change as children progress through different stages of development. From newborns to toddlers and school-aged children, the methodologies, tools, and objectives of hearing tests evolve to capture the intricacies of auditory development at various milestones. With a better understanding of the nuanced approaches and considerations that…
Sublingual Immunotherapy (SLIT) Explained
When we talk about allergies and their management, the first thing that often comes to mind is allergy shots or injections. However, for those who are averse to needles or find the regular visits to a doctor’s office inconvenient, there's another effective option known as Sublingual Immunotherapy (SLIT). Understanding Sublingual…








 Make an Appointment
Make an Appointment Morpheus8 – The groundbreaking advancement in non-surgical aesthetic treatments, blending the power of microneedling with radiofrequency (RF) technology to rejuvenate and remodel the skin. This innovative, non-surgical skin care procedure provides impressive anti-aging results with minimal downtime. Treatments can be tailored to target individual skin concerns, whether it’s wrinkles, acne scars, stretch marks or uneven texture.
Morpheus8 – The groundbreaking advancement in non-surgical aesthetic treatments, blending the power of microneedling with radiofrequency (RF) technology to rejuvenate and remodel the skin. This innovative, non-surgical skin care procedure provides impressive anti-aging results with minimal downtime. Treatments can be tailored to target individual skin concerns, whether it’s wrinkles, acne scars, stretch marks or uneven texture.